Terminology – Adhesives
-
Adhesive; an adhesive is a non-metallic substance which is capable, after changing from a liquid into a solid state, to bond materials together by means of adhesive-, and cohesive forces.
-
Adhesion; bonding to a substrate by attraction between molecules and/or atoms of different substances (adhesive /substrate)
-
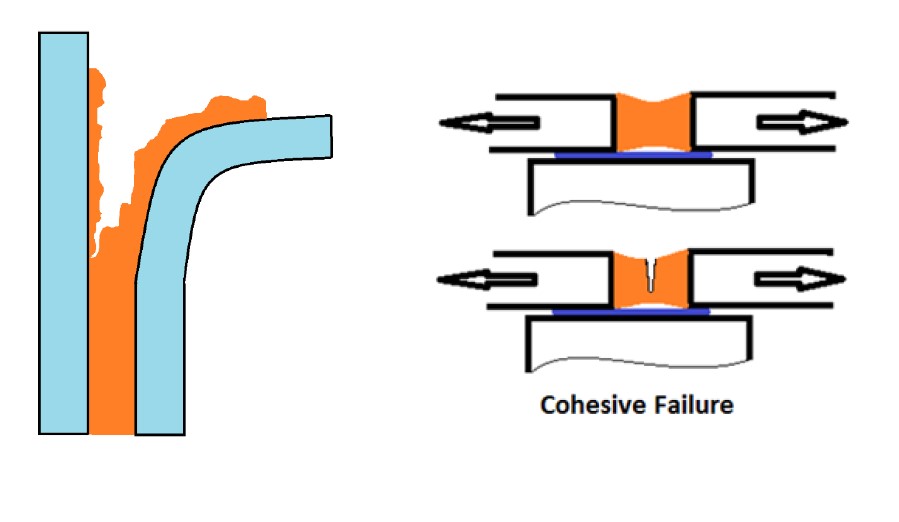
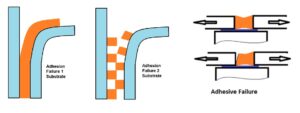
Adhesive failure; Rupture of an adhesive bond, such that the separation appears to be at the adhesive substrate interface. There is no adhesive left at the substrate
-
Cohesion; attraction between molecules and/or atoms within the same substance (adhesive)
-
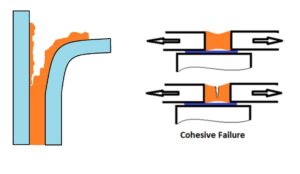
Cohesive failure; Rupture of an adhesive bond, such that the separation appears to be within the adhesive.
-
Wetting; is the contact between a liquid and a solid surface. Wetting of a surface is important for the degree of adhesion to different materials. In case of a low surface tension [mN/m] of the substrate the fluid will form a compact droplet (A) on the surface (S). (e.g. on PE, PP and Teflon). In case of a high surface tension of the substrate the fluid (C) will spread to cover a larger area of the surface. The surface tension of a substrate can easily be measured by bringing a droplet of water to the surface. In case water stays in a droplet (A) the surface tension of the substrate is low. In case the droplet flows over the surface the surface tension of the substrate is high. In case of a high surface tension the adhesive will easily flow over the surface as well and will give a good adhesion
-
Contact adhesive; an adhesive which dries to a film that is tack-free to other materials but not to itself. The adhesive is applied to both surfaces to be joined and dried (evaporation of solvent or water from the adhesive) at least partially. When pressed together at light to moderate pressure, a bond of high initial strength results immediately. It is usually not necessary to apply pressure for a long time.
-
1C adhesive; typical characteristic for this 1-part chemical curing or reactive adhesives is that the second ‘component’, needed for curing, is taken from the atmosphere and/or environment (moisture, oxygen, UV, reactive groups from the to bebonded substrates).
-
2C adhesive; typical characteristic for this 2-part chemical curingor reactive adhesives is that the second component (B-component or hardener) must be mixed in to start the curing reaction.
-
Transfer method; the adhesive is applied to one of the to be bonded materials. The second substrate is placed in the wet adhesive and removed immediately. After evaporation of solvent from the adhesive both substrates are joined together and pressed firmly.
-
Tensile strength; is a measurement to classify the mechanical properties of an adhesive. The tensile strength is the point at which an adhesive, under the stress of an applied force, snaps, breaks or can no longer maintain its structural integrity. It is, in other words, the amount of force the material can withstand without breaking. The maximum load at which this happens is called tensile strength. The unit of tensile strength is MPa (megapascal) or N/mm².
- 1 Pa = 1 N/m², (± 10N equals 1 kg) => 1Pa = 0,102 kg/m²
- 1MPa = 1.000.000Pa
- 1MPa = 102.000 kg/m², or
- 1.000.278 N/m² or 1N/mm² (0.102kg/mm²)
-
Shear strength; maximum stress that a material can withstand before failure in shear. Hereby the force is parallel to the bonded area. The unit of shear strength is MPa (megapascal) or N/mm².
(1 Pa = 1 N/m² or 1MPa = 1N/mm²).
-
Peel strength; a bond is not resistant against peel and constant load. In case of peel a large force is applied to a very small adhesive area. This will overload the adhesive and a failure will occur. Peel forces (load) always need to be prevented.
-
(High) tack; or initial grab is the ability of an adhesive to keep vertical bonded objects in place without support. Initial grab is expressed in kg/m² (or pound/ft²) => weight of object vs. area.
There is no relation between initial grab and e.g. tensile strength or shear strength.
Example:
Initial grab: 100 kg/m² = 0.0001 kg/mm² = 0.001N/mm² (ca. 10N = 1kg)
If we take a look at final end strength, expressed in N/mm² (or MPa), the difference between initial grab and tensile- or shear strength becomes clear directly End strength: 1N/mm² (1Mpa) = 0.100 kg/mm² = 100.000 kg/m² (1kg = ca. 10N)
-
Elasticity (elastic adhesive); A material is said to be elastic fi it deforms under stress (e.g. external forces), but then returns to its original shape when the stress is removed. An example of an elastic material is rubber.
-
Density; indicates how much mass is present in a certain volume. Traditionally density is indicated by the Greek letter “?” (rho). The formula of density is: Density is expressed in kg/m³ (SI unit). It is also sometimes given in the cgs units of grams per cubic centimetre (g/cm³). => 1000 kg/m3 = 1 g/cm3 = 1 g/ml
-
Total solid content (TSC); is the mass percentage of non ‘volatile’ compounds which are left as a residue after complete drying of a certain amount of material. Depending on the product the total solid content can exist of; polymer, fillers, additives, resin, plasticizer,…… In case of sealants the loss of mass percentage (e.g. the evaporation of water from acrylic based sealants) also means a proportional shrinkage.
-
Viscosity; indicates how liquid a product (e.g. an adhesive) is. Or more precisely: a measure of the resistance of a fluid which is being deformed by either shear stress or extensional stress. The unit of viscosity is Poise (or Pa.s. = Pascal * second). To compare the viscosity of liquids against water millipascal/second (mPa.s.) or centipoise (cP) is used (1 cP = 1 mPa.s.). The higher the value the higher the viscosity. Water is a liquid with a low viscosity (approx. 1 mPa.s. or 1 cP). Honey for instance is a liquid with a high viscosity. Viscosity is dependent on temperature; most liquids are lower viscous at high temperature as they are at lower temperature.
-
Thixotropy; the property of a product (fluid) to show a time-dependent change in viscosity; the longer the fluid undergoes shear stress, the lower its viscosity. After shear stress stops the product returns to its original viscosity. Examples of thixotropic fluids are
-
Ketchup; The “common” method (inverting the bottle and hitting the bottom withthe heel of the hand) will cause the ketchup to begin flowing over itself
-
Paint; Modern alkyd and latex paint varieties are often thixotropic and will not run off the painter’s brush, but will still spread easily and evenly, since the gel- like paint “liquefies” when brushed out
-
-
Tack; the property of an adhesive that enables it to form a bond of measurable strength immediately after adhesive and substrate or another adhesive layer are brought into contact under low pressure.
Tack is the property of a pressure sensitive adhesive which allows it to adhere to a surface under very light pressure
Examples of products with a high tack are; labels and tape
-
MFFT (Minimum Film Formation Temperature); is the lowest temperature at which an emulsion, dispersion or water-based adhesive will uniformly coalescence (merging of two drops into a single larger drop) and a continuous film is formed. Below this temperature ‘coagulation’ of the separate polymer particles in a water-based adhesive will not take place and therefor the adhesive will not cure
-
Open time; The maximum amount of time that an applied adhesive can be exposed to air before joining both substrates. If the open time is exceeded before the substrates are joined, the adhesive will lose its ability to bond to the second substrate. At this point an adhesive is because of evaporation of water or solvent or because of chemical curing that dry that an enough wetting will not take place.
-
Pot-life; the time that a mixed adhesive is still low viscous enough to be applied. Pot-life is partly depending on temperature (a higher temperature will give a shorter pot-life and a lower temperature will give a longer pot- life). Beside temperature also the number of mixed components is determining the pot-life. The more adhesive that is mixed, the more an adhesive’s pot life decreases. The heat which is released during the reaction between A and B component has an accelerating influence on the curing speed. The more mixed adhesive the more heat is released. Accelerating the curing speed results again in more reaction heat. Therefore, the pot-life is generally measured on 100gr. mixed A+B component.
-
Clamp time; the minimum required time to clamp bonded parts together. From this moment on the adhesive is cured enough to replace or machine the bonded parts. Clamp time is depending on the amount of adhesive, porosity of the bonded materials, temperature and relative humidity.
-
Curing time; is the total (process) time needed to reach a 100% strong bond. Generally, the strength buildup follows the “S” curve below.
-
Temperature (heat) resistance; at a (long-term) exposure to a certain temperature an adhesive will lose its mechanical properties. The temperature at which this happens is different for every polymer. The highest measured temperature at which an adhesive still has its original mechanical properties is called Temperature (or heat-) resistance. Generally, thermoplastics (materials that become weak when heated) are having a low Temperature resistance. Thermosetting materials (materials that are irreversibly cured to a stronger form) generally are having a high temperature resistance. Thermoplastic adhesives; basically, all water borne adhesives such as PVAc- (or white wood adhesives) and solvent based adhesives such as Polychloroprene (Neoprene) adhesives. Thermosetting adhesives; e.g. 2C PU-adhesives, 2C epoxy adhesives. Beside a high temperature resistance, they generally also have a good water and chemical resistance.
-
Water resistance; at a (long-term) exposure to water an adhesive will lose its mechanical properties. The degree of resistance is tested according to EN204. Based upon the test results adhesives can, according to EN204, be classified in 4classes
- D1; Interior applications in which the moisture content of the wood does not exceed 15%
- D2; Interior applications with occasional short-term exposure to running or condensed water and/or to occasional high humidity provided, the moisture content of the wood does not exceed 18%
- D3; Interior applications with frequent short-term exposure to running or condensed water. Interior applications with long-term exposure of the bonds to high humidity. Exterior applications which are
not exposed to weather - D4; Interior applications with frequent long-term exposure to running or condensed water. Exterior applications with exposure to weather influences but with protection by an adequate surface coating.
Want to save this guide? Download the PDF version here